The rate of diffusion of two gases X and Y is in the ratio of 1:5 and that of Y and Z in the ratio of I: 6. The ratioof the rate

The ratio of rate of diffusion of gases A and B is 1 : 4. If the ratio of their masses present in the mixture is 2 : 3, what is the ratio of their mole fraction ?
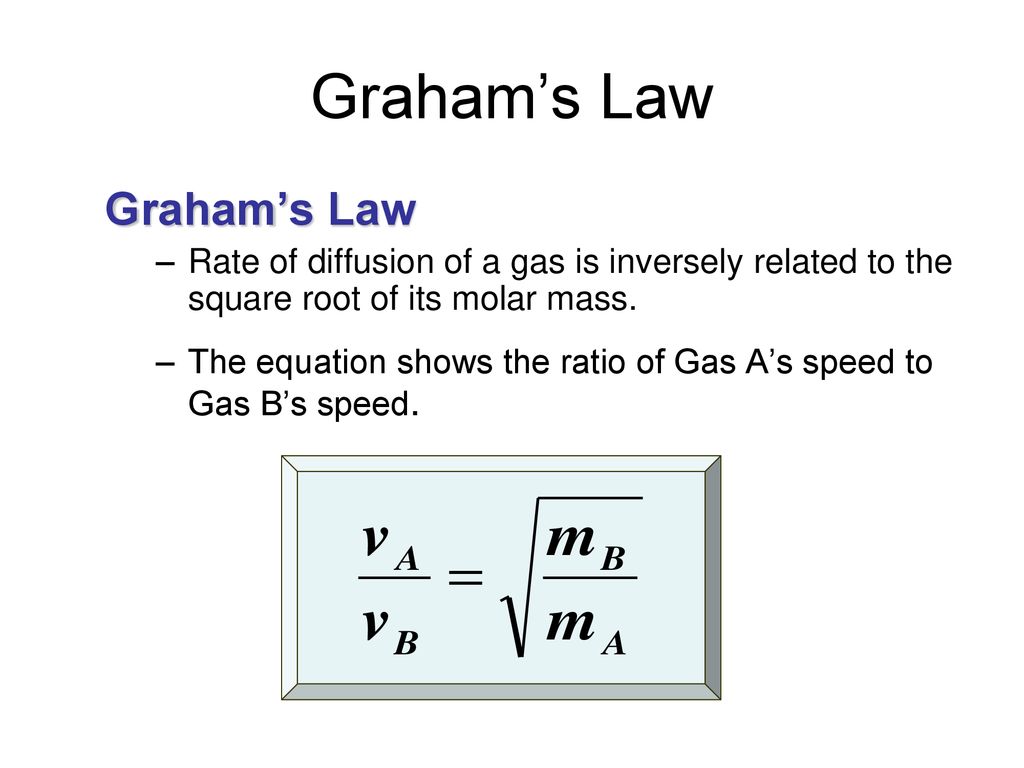
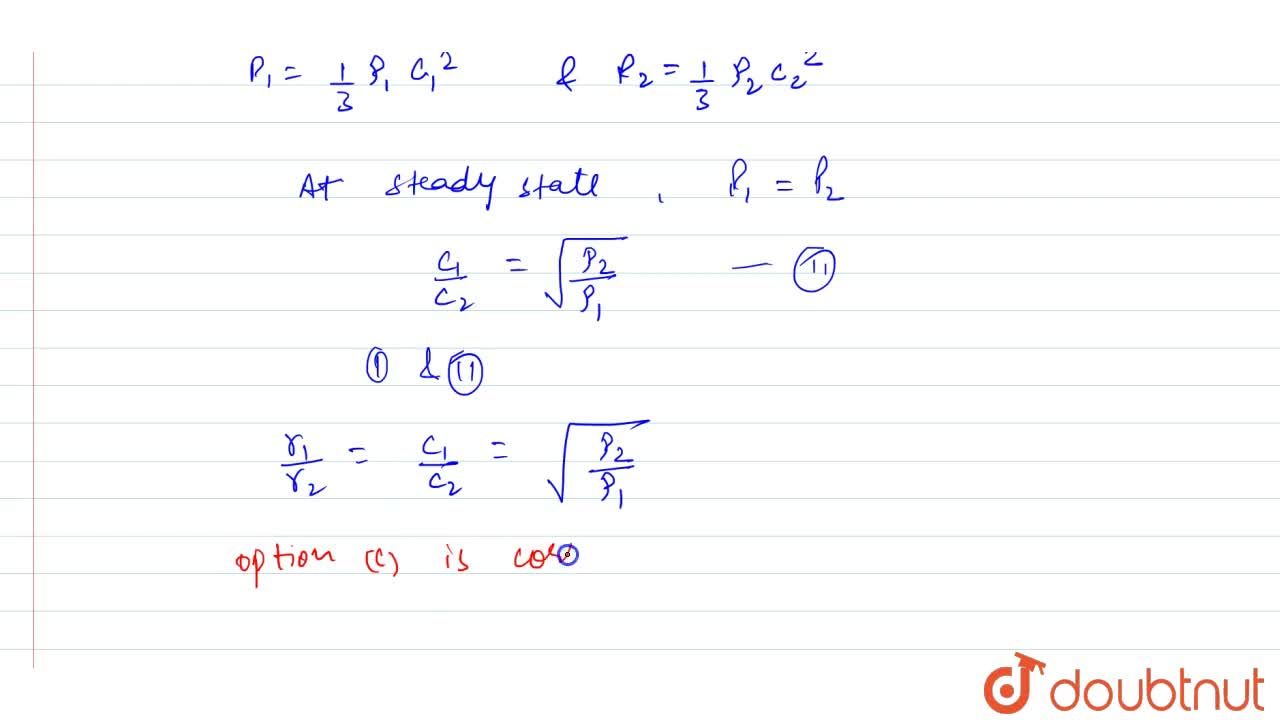
Both rate of diffusion and effusion is inversely proportional to root M because of the common fact that (1) Diffusion and effusion are same (2)Molecular speed directly proportional to 1/root M (3)Both
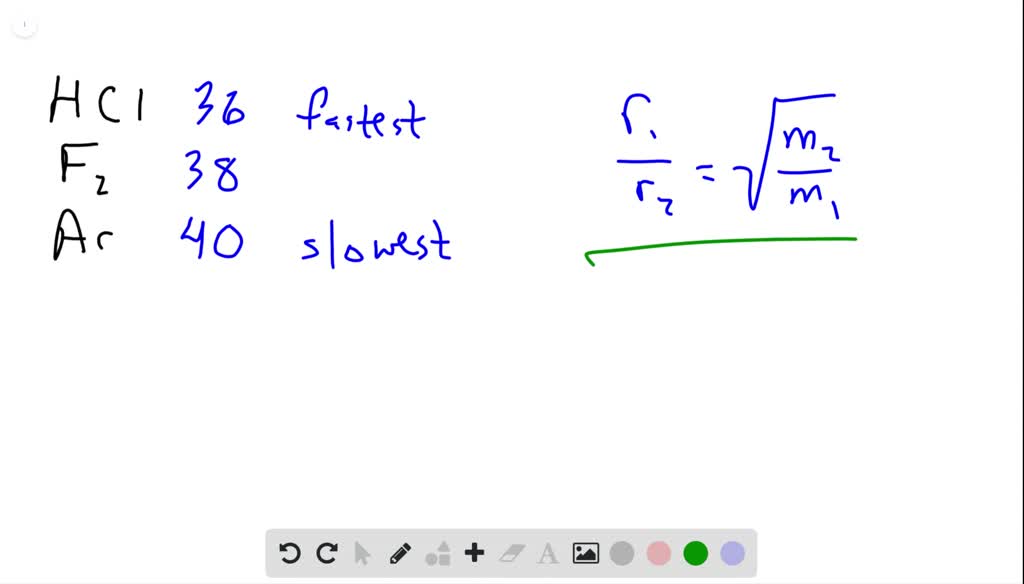
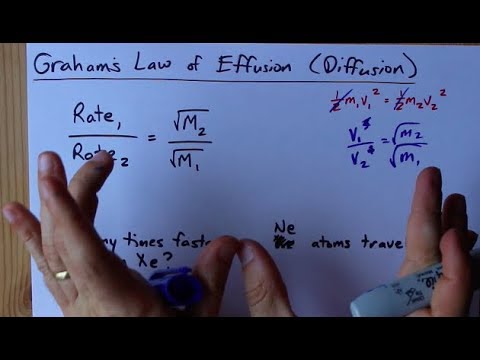
SOLVED:Rank the following gases in order of their speed of diffusion through a membrane, and calculate the ratio of their diffusion rates: HCl, F2, Ar .

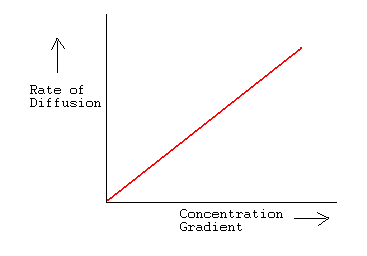
SOLVED:The rate of diffusion of a gas is proportional to: (a) (P)/(√(d)) (b) (P)/(d) (c) √((P)/(d)) (d) (√(P))/(d)

The ratio of rates of diffusion of gases X and Y is 1:5 and that of Y and Z is 1:6. The ratio of rate of diffusion of Z and X is:

The rate of diffusion of a gas is proportional to | 11 | STATES OF MATTER | CHEMISTRY | R SHARM... - YouTube