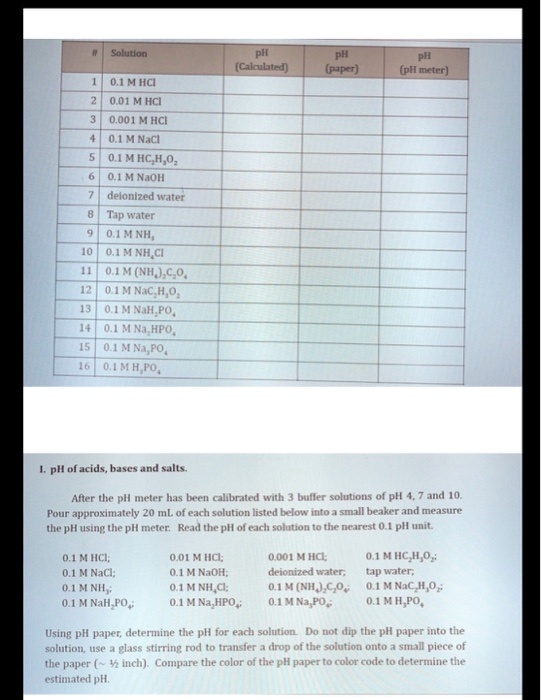
10 ml of a solution having PH =4 is added with 990 ml of 0.1M NaCl solution. PH of resulting solution - Brainly.in

A weak acid HX has the dissociation constant value of 1 × 10^-5 M . It forms a salt NaX on reaction with NaOH . The percentage degree of hydrolysis of 0.1 M solution of NaX is:
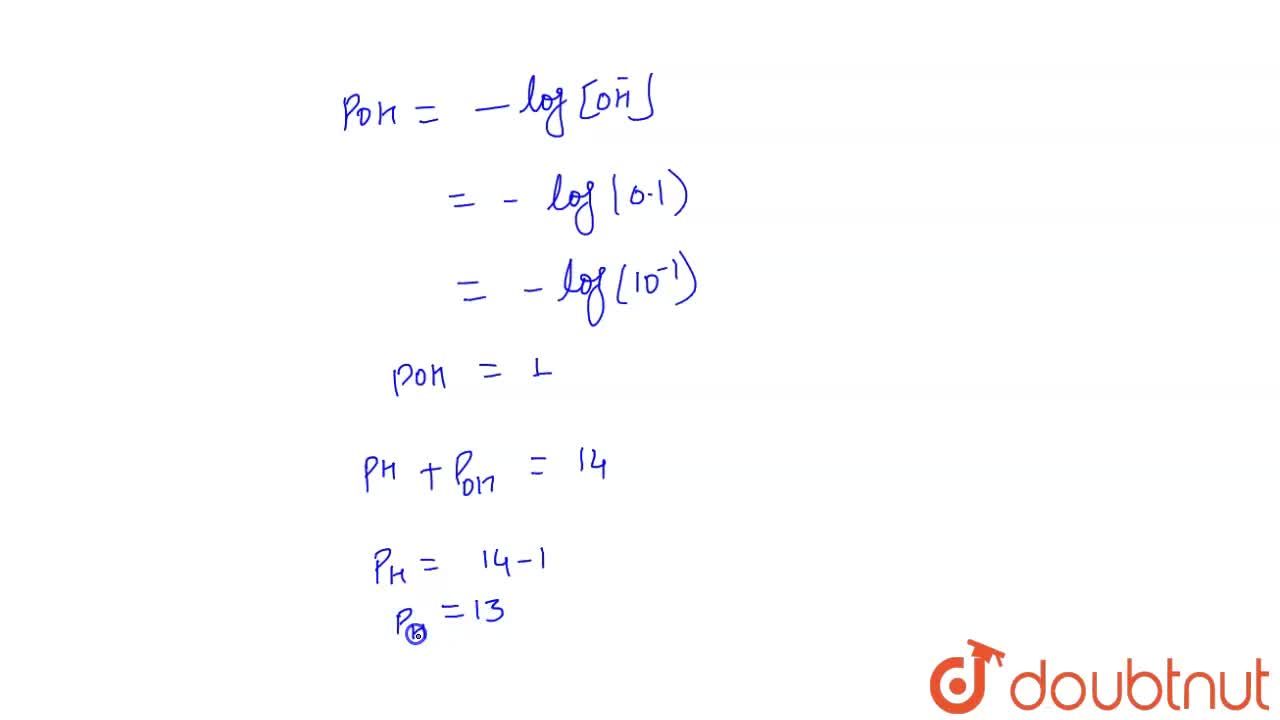
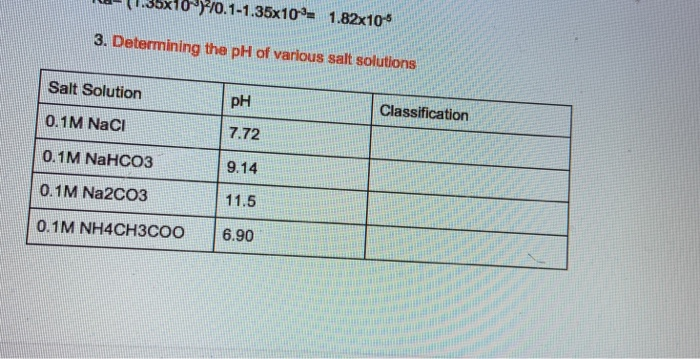
Calculate the pH of 0.5 of 1.0 M NaCl solution after electrolysis when a current of 5.0 ampere is passed for 965 seconds.
The pH of 0.1M solution of the following salts increases in the order - Sarthaks eConnect | Largest Online Education Community