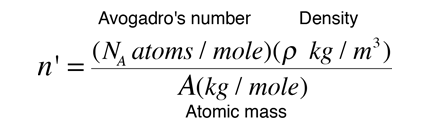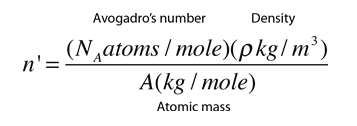Welcome to Chem Zipper.com......: In diamond, carbon atoms occupy FCC/CCP lattice point as well as alternate tetrahedral voids. If edge length of unit cell is 3.56pm, the radius of carbon atom is ?

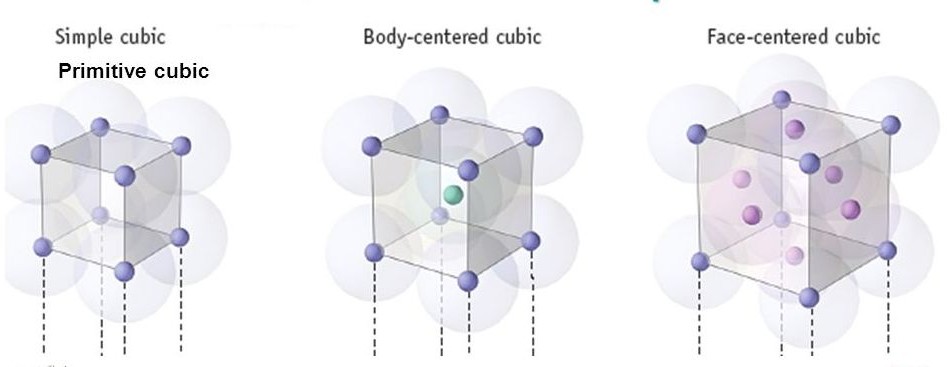
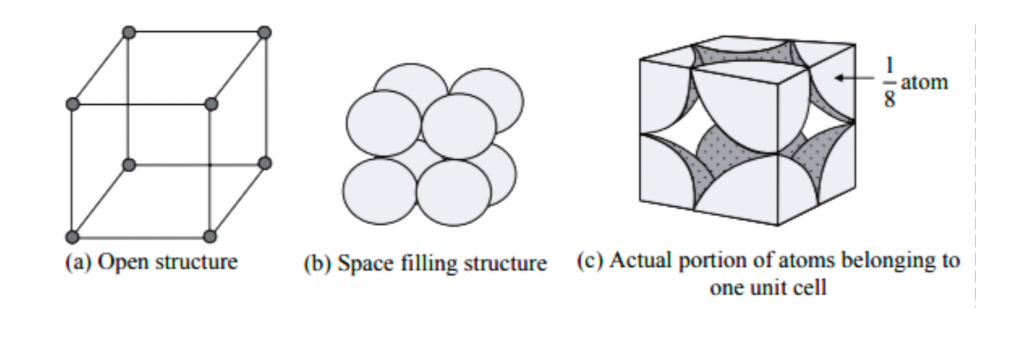
SOLVED:What is the number of atoms per unit cell for each metal? (a) Polonium, Po (b) Manganese, Mn (c) Silver, Ag

Density of a unit cell is same as the density of the substance. If the density of the substance is known, number of atoms or dimensions of the unit cell can be
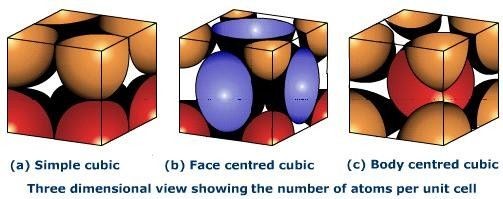
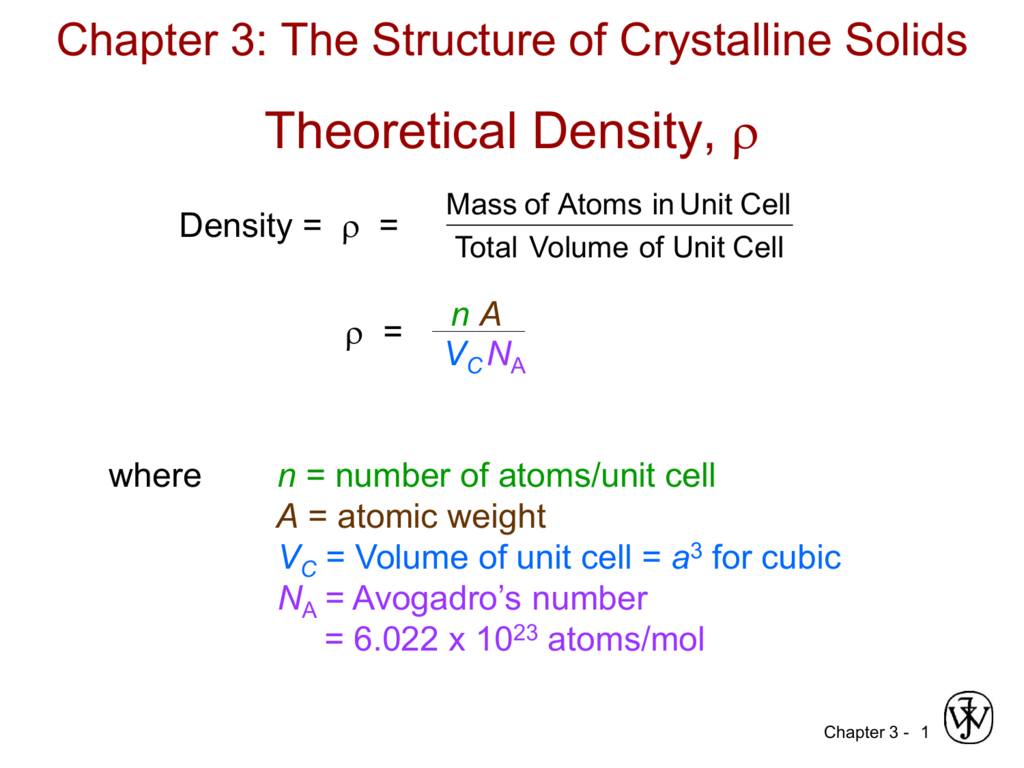
What is Atomic Packing Factor (and How to Calculate it for SC, BCC, FCC, and HCP)? – Materials Science & Engineering

A domain in ferromagnetic iron is in the form of a cube of side length 2 mu m then the number of iron atoms in the domain are (Molecular mass of iron =

An element (atomic mass = 31) crystallises in a cubic structure. The density of the metal is 5.4g*cm^(-3). The number of unit cells is 3.1g of metal is 6.022xx10^(22). The number of
What is Atomic Packing Factor (and How to Calculate it for SC, BCC, FCC, and HCP)? – Materials Science & Engineering
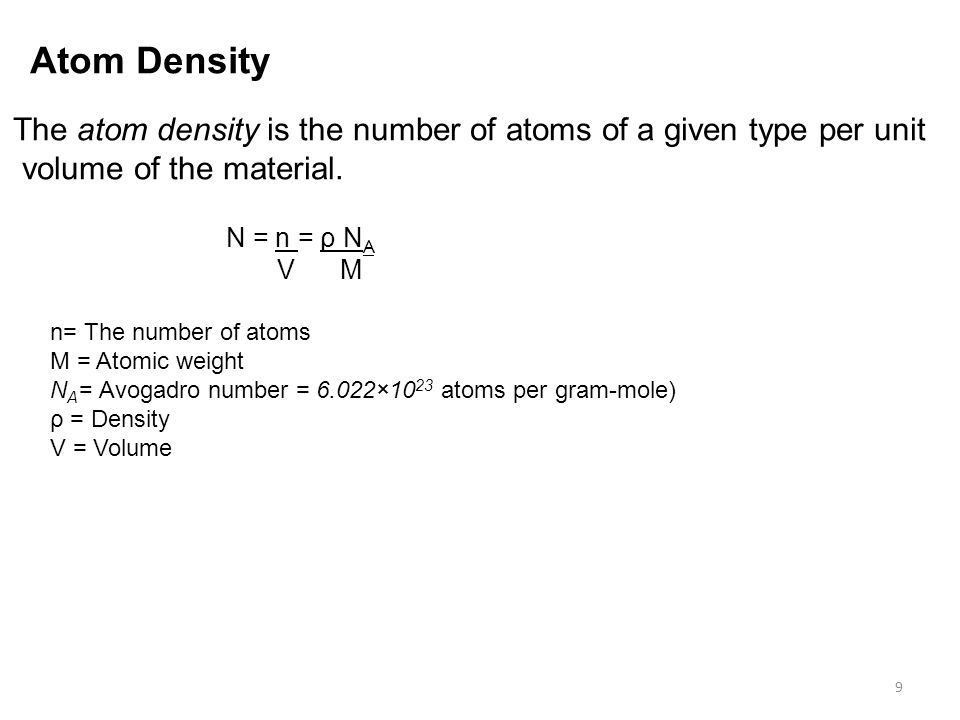
Laboratory 05 Periodic Trends : Densities in the Chromium Family of Transition Metals. - ppt video online download
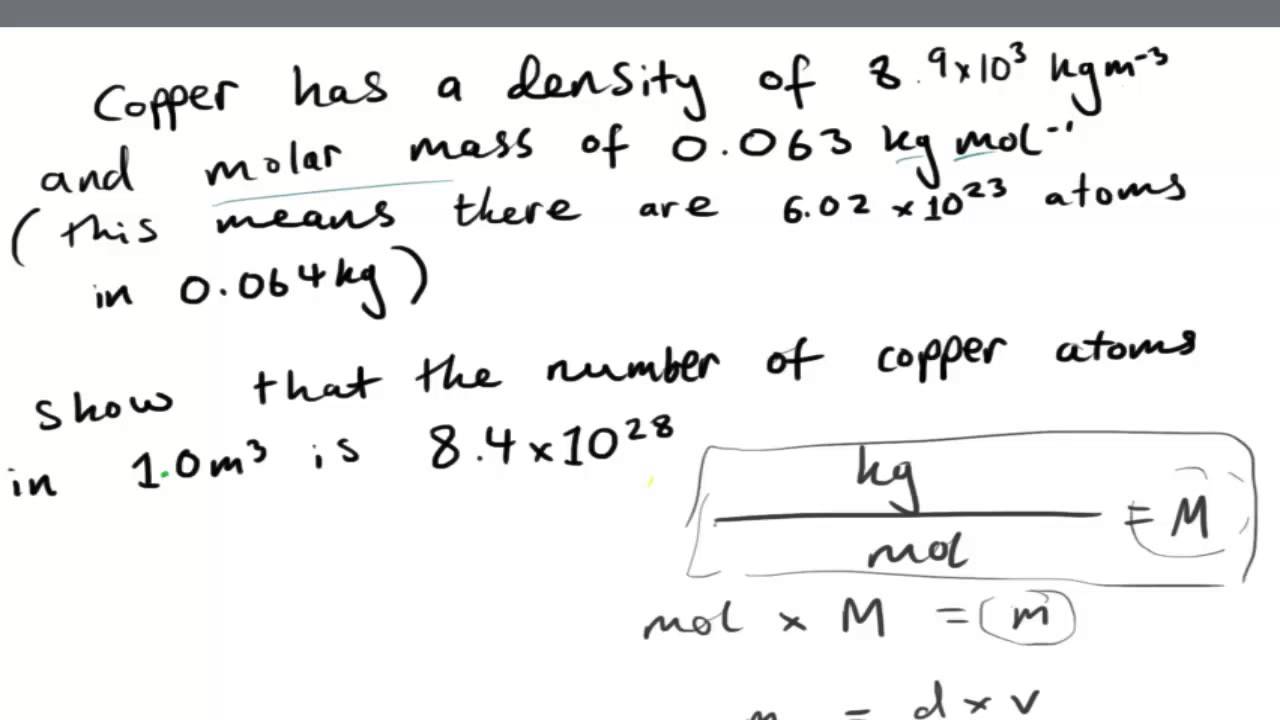
Working out the Number of Atoms in a given volume of Copper from Density/Molar Mass- AS Physics - YouTube