
Molar Mass & Molarity. Molar Mass Mass in grams of one mole of an element or compound. Numerically equal to the atomic weight of the element or the sum. - ppt download
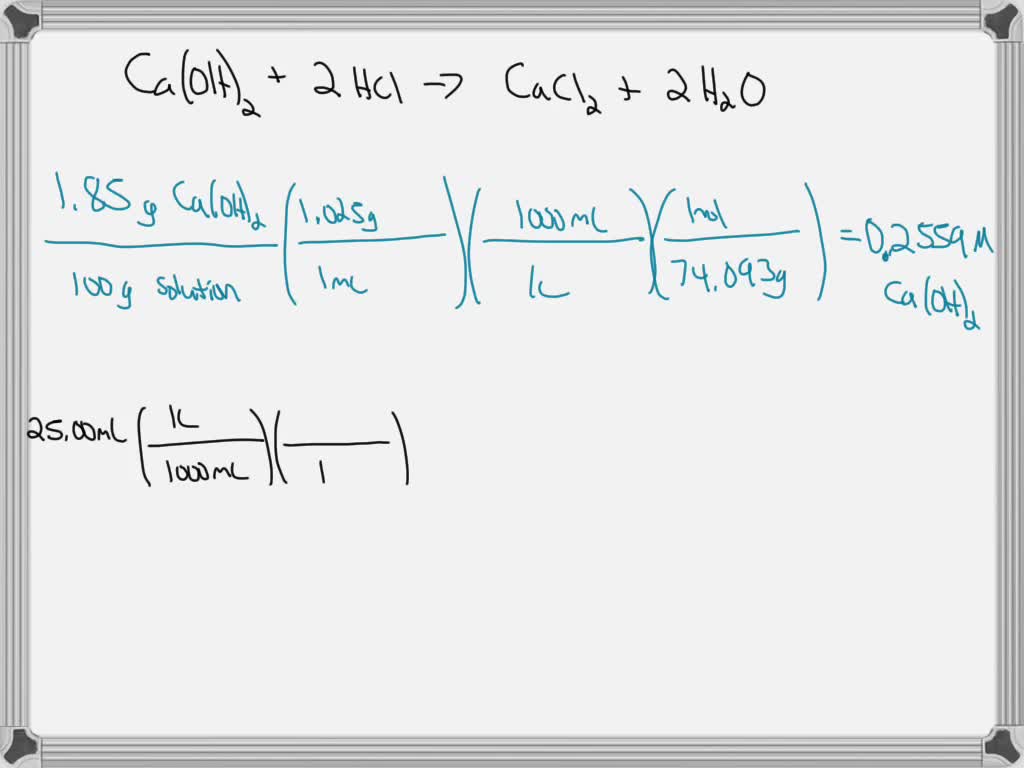
SOLVED: How many mL of 1.5 M HCl solution is required to completely neutralize 20.00 mL of 10.0% calcium hydroxide solution (you can assume density is 1.00 g/mL), please show your work

Degree of Surface Coverage of Aluminium in 1.0M HCl and 1.5M HCl by HEC | Download Scientific Diagram

A drop ( 0.05 mL) of 12M HCl is spread over a thin sheet of aluminium foil (thickness 0.10 mm and density of Al = 2.70 g/mL). Assuming whole of the HCl

SOLVED: Compute for the molarity of the solution when you mix 0.05 L of 2 M hydrochloric acid solution and 0.015 L of 1.5 M hydrochloric acid solution.

SOLVED: Find the final volume, in mL, of each of the following examples: A 1.5 M HCl solution prepared from 20.0 mL of a 6.0 M HCl solution A 2.0 % (m/v)

A drop ( 0.05 mL) of 12M HCl is spread over a thin sheet of aluminium foil (thickness 0.10 mm and density of Al = 2.70 g/mL). Assuming whole of the HCl

Question 2.4 please. If 250ml of 1.5M Ba(OH)2 solution mixes with 250ml of 2.0M of HCl solution, - Brainly.com












![BT021-1] 1.5M Tris-HCl, pH 8.8 | Biosolution BT021-1] 1.5M Tris-HCl, pH 8.8 | Biosolution](http://biosolution.cafe24.com/wp-content/uploads/2015/05/BT021-1-1-5M-Tris-8-8.jpg)